Pharmaceutical Packaging Design: Safety, Compliance, and New Technology
The pharmaceutical industry is strict and controlled in terms of the safety and compliance of its products and medicines, and with good reason!
Quality, protection and care must never be compromised, especially when the health and well-being of individuals are at stake.
These requirements apply not only to the products themselves, but also to the design of medicine packaging.
When it comes to pharmaceutical products, the design of the packaging and the labelling are equally important.
The design of pharmaceutical packaging should prevent contamination by water, light, and dust, and the labels should be readable, concise, and accurate.
Failure to meet these standards can lead to business failure, so in this article, we will discuss the key factors you must consider to remain in business and maintain the high standards of the pharmaceutical industry.
Nevertheless, satisfying these requirements has never been more convenient for the packaging of medical devices!
Quality control, traceability and transparency have never been more easily attained than with the introduction of smart packaging design.
However, before examining this trend, we will first take a closer look at the requirements for medical packaging.
- 1. Medical Devices and Packaging Regulatory Bodies
- 2. Designing safe and compliant medical device packaging
- 3. Sterility and protection are key requirements for medical device packaging
- 4. The human factor and labelling in the design of pharmaceutical packaging
- 5. Supply chain considerations
- 6. Medical Device Packaging Testing
- 7. New trends in the design of medicine packaging
- 8. Never before has medical packaging design been easier
1. Medical Devices and Packaging Regulatory Bodies
We will only proceed to testing and other considerations of your pharmaceutical packaging design once we have discussed the design in detail. However, it is important to start with the broad strokes first.
As mentioned earlier, the medical industry is highly regulated. This is why, before companies can consider how to fulfil end users' needs, they must comply with the rules established by the government and international regulators.
The key organisations that you need to comply with with regard to medical device packaging design requirements are the US Food and Drug Administration (FDA) and the International Organization for Standardization (ISO).

US government regulation is the FDA, whereas the ISO is an international non-governmental organisation consisting of numerous national standard bodies.
Why is it important to ensure compliance with FDA and ISO regulations?
Although the ISO is a non-governmental organisation, it plays a crucial role in promoting trade worldwide. It operates in 167 countries, establishing universal standards.
These standards ensure that all products meet the minimum global standard.
Regardless of whether your medical device is categorised as Class I, II or III, these regulatory bodies have a long list of requirements that must be met.
These regulations cover labelling standards, data integrity, usage, sterilisation, age testing, shelf life, and international distribution.
Although the full list of regulations and requirements for each class of medical device is more detailed and comprehensive, most organisations and manufacturing companies use in-house or outsourced departments to monitor, compile and keep up to date with these requirements.
Identifying medical needs at an early stage of development reduces the risk of defects and expensive recalls. Therefore, make sure that you comply with FDA and ISO standards.
Now that you are familiar with the FDA and ISO, and their role in establishing standards for medical products and their packaging design, we can turn our attention to how medical packages can be designed to comply with these standards.
2. Designing safe and compliant medical device packaging
As mentioned briefly above, there are a number of regulations governing the design of medical product packaging, especially with regard to labelling, sterility and barrier requirements.
However, before considering these factors, it is important to discuss your preferences with your preferred packaging partner.
Depending on the characteristics of the package and the films selected, the manufacturing process may change significantly due to the various requirements of machine models, applications and sealing elements.
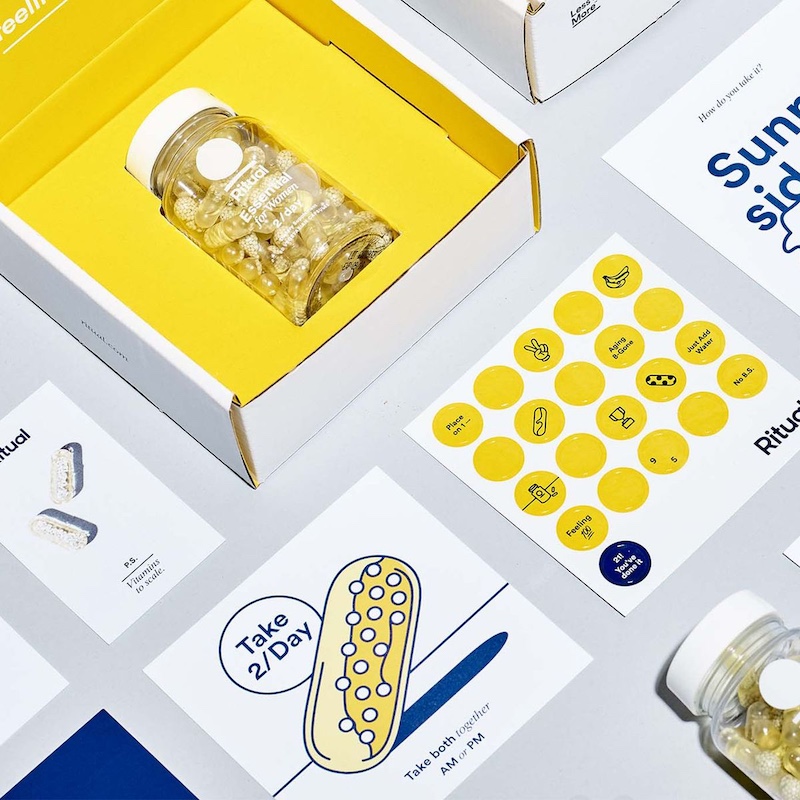
Communicate your preferences for materials and packaging with your chosen packaging partner to avoid issues that may require you to redo all of your pharmaceutical packaging design in the future.
Remember to consider aspects such as appearance versus functionality, potential resource shortages, prices, sealing techniques, recyclability and other factors.
The drive towards sustainable packaging is gaining momentum, and this must be taken into consideration with medical device packaging too.
While your packaging partner will not decide what your packaging needs in terms of the medical aspect, they will assist you in keeping track of these considerations and provide guidance on what needs to be addressed.
3. Sterility and protection are key requirements for medical device packaging
After discussing the materials and other preferences with your packaging partner, the next important considerations are sterilisation and barrier properties.
In terms of design, the medical package material should be able to withstand the sterilisation process, be sterile and act as a viable barrier to contaminants.
It should be noted that sterilisation methods can differ, including ethylene oxide (EO), radiation or steam, and materials respond differently to each option.
Make sure that the material you have selected is compatible with the sterilisation process and the necessary barriers and protection.
Medical devices may require certain barriers, including:
- Moisture
- Odours/vapours
- Acids/bases
- Microbial
- Temperature
Once again, the selected material will determine what your package will be shielding from, so it is important to communicate with your packaging partner and decide on your materials.
4. The human factor and labelling in the design of pharmaceutical packaging
Once you have selected the right material and ensured that it is compatible with sterilisation and provides the necessary protection against contaminants, you need to consider the human factor.
While it is important to shield your device against contaminants, accessibility is also important.
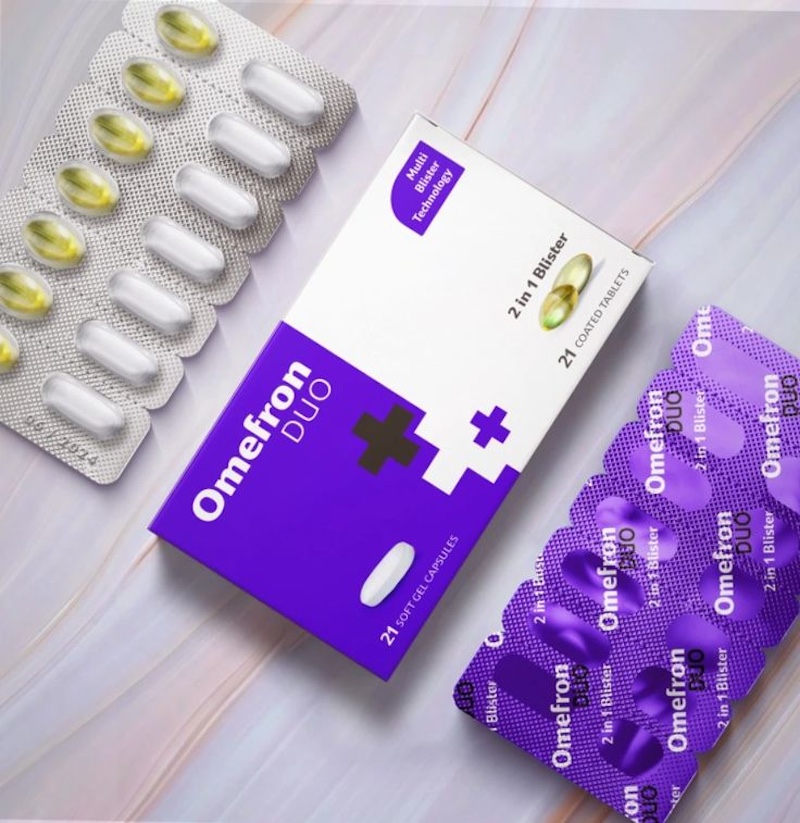
The design of your medical package must take the end user into account and be easily accessible to them in their environment. It should also be sterile when opened.
However, note that some devices may require additional design considerations to ensure adults can access them easily, while children, seniors, and other disabled individuals cannot.
Consider all the possible scenarios in which your medical device could be used and base your packaging design on these to allow for emotional, environmental or physical external conditions.
Proper labelling and instructions will also help with this.
Clear and concise instructions are extremely helpful for the end user, and appropriate labelling enables clear definitions of what your device can do, ensuring a successful product launch and preventing misuse by the end user.
Failure to include such labels, or including incorrect labels, is one of the main reasons why the FDA recalls products. Therefore, make sure your labels are correct!
Coordinate with the regulatory, quality assurance and manufacturing teams throughout the process, applying the labels prior to the testing phase to enable prompt corrections.
5. Supply chain considerations
When designing your medical device packaging, the last factor to take into account is the supply chain.
As mentioned above, other medical devices and medicines have different requirements throughout their lifecycle, and the supply chain is no exception.
When designing your medical device package, you should consider the transportation and storage needs of your device.
Is it temperature sensitive? Light sensitive? Should the device keep records of its movements, or should tracing be adopted for the package? Will your package survive rough transportation that is likely to cause contamination?
These are all questions that need to be answered when designing pharmaceutical packaging, and testing is essential for resolving such problems.
6. Medical Device Packaging Testing
Once all considerations have been taken into account and the designs have been implemented, your medical device package must be subjected to rigorous testing to determine whether the sterilisation, barriers and other systems in place are safe and effective.
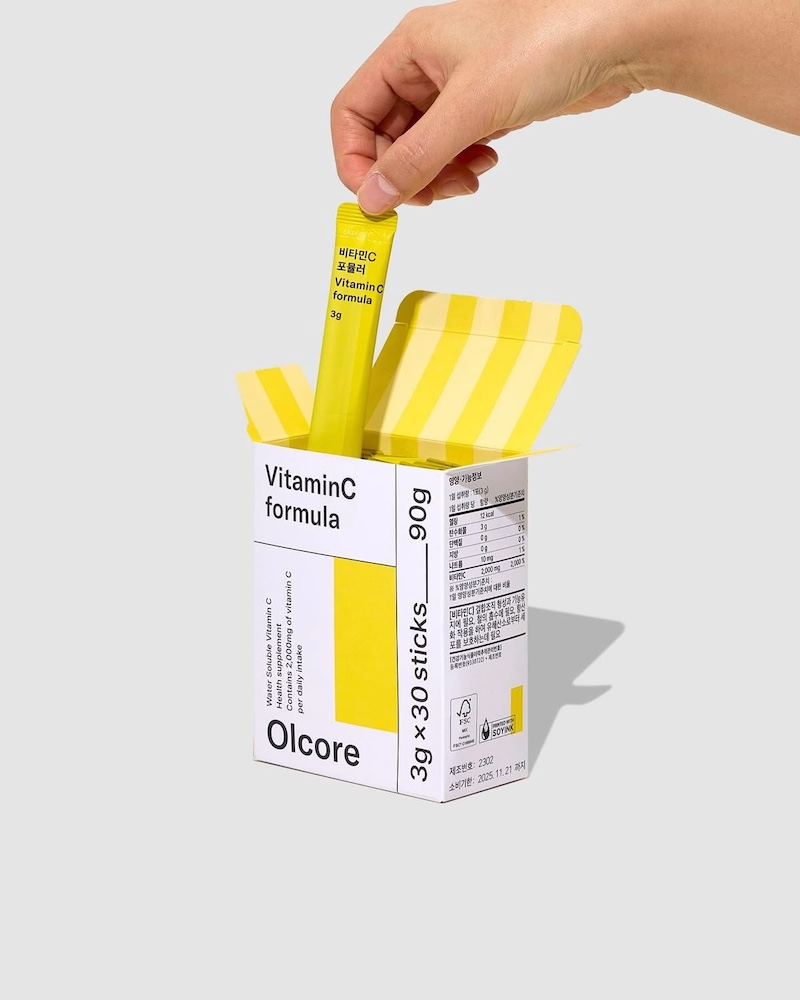
From manufacturing to transit and storage, and even until the consumer receives it, your pharmaceutical packaging must comply with FDA and ISO provisions.
To facilitate this process, you can use certified third-party testing laboratories to carry out tests such as performance testing, accelerated ageing testing, temperature testing, humidity testing, seal testing and sterile integrity testing in controlled conditions to ensure accurate results.
Examples of requirements that must be met during medical device packaging testing include, but are not limited to:
- Packaging systems must be capable of safeguarding their contents if they are subjected to dangerous conditions during handling, distribution or storage.
- Seals and packages should be strong and safe under mechanical stress.
- The package's integrity should ensure that the barrier remains sterile until the package is opened.
- The sterile barrier system must not break during the product's projected shelf life.
7. New trends in the design of medicine packaging
Despite the many requirements and regulations surrounding medical device packaging, and the numerous factors to consider when designing a package that meets these high standards, keeping up with packaging trends can help to alleviate some of the stress involved.

Smart packaging and tracking technologies are becoming increasingly popular, making it much easier to comply with the demanding standards of the medical industry.
Sophisticated sensors and indicators have been designed to assist in checking the status of a medical product during transportation and storage, and can even enhance its shelf life.
Supply chains have also become more transparent thanks to technologies such as RFID tags and tracking stickers, which give companies real-time visibility of their medical device packages' logistics and transportation.
Smart packaging technologies enhance the end-user experience by making quality, transparency, and relevant information accessible quickly and efficiently.
Given the growing use of smart packaging in the medical and other fields, it may be worth considering incorporating such technologies into your medicine packaging designs.
Stay ahead of the curve and help mitigate some of the challenges involved in creating packages that satisfy FDA and ISO standards.
8. Never before has medical packaging design been easier
Regardless of the purpose or function of your medical device, the FDA and ISO will never compromise on pharmaceutical packaging and products.
This is hardly surprising when the health and well-being of individuals are at stake.
However, despite the fact that standards will not decrease and companies will continue to comply with rigorous requirements, pharmaceutical packaging design is finally becoming more accessible.





About Us
Can I get a sample?
Yes, currently sample free for reference, only need to pay the delivery charge. Customized sample charge sample fee and delivery cost.
OEM Services
What is OEM packaging, and how can Winpack Printing help?
OEM (Original Equipment Manufacturer) packaging refers to custom-designed packaging solutions tailored to a brand’s specifications. At Winpack Printing, we provide end-to-end OEM services, including design, proofing, printing, and production, to help you create high-quality, market-ready packaging that aligns with your brand identity.
Do you offer eco-friendly and sustainable packaging options?
Yes! We provide environmentally friendly packaging solutions, including recyclable, biodegradable, and FSC-certified paper materials. Let us know if you’re looking for sustainable options.
Can I request a sample before mass production?
Yes, we provide sample proofing before mass production to ensure that the design, materials, and quality meet your expectations. We encourage testing and approval before proceeding with full-scale manufacturing.
Rigid Lift-off Box
Can I order a sample of my rigid box?
Yes, we strongly recommend that you do so, we provide a variety of sample types to meet different usage situations and help you guarantee the best results!




Start Your Packaging Journey Today
Discover Our Curated Collection of Bespoke Packaging Cases & Tailored System Solutions
© 2025 WINPACK PRINTING. All rights reserved






















Wenhua Printing packaging
winpackprinting
Winpack Printing